No longer will researchers have to choose between studying a single human brain as a mosaic of fragmented images or a distant, pixelated view of large structures.
The new imaging platform developed by the US team instead seamlessly combines the finer details of brain cells, their connections and content, with whole-brain maps of the entire networks of neurons that maintain the brain’s overall architecture.
These elements of brain biology exist at very different scales, from nanometer synapse gaps to centimeter regions of the brain, which until now required multiple samples from multiple brains to analyze using different technologies on different platforms.
In the first demonstrated use on human tissue, imaging two whole brains, the platform revealed distinct changes in the brain of one person with Alzheimer’s disease.
The new platform includes three essential elements for cutting, processing and then imaging brain tissue with “unprecedented resolution and speed,” according to the research team that developed it, led by Kwanghun Chung, a chemical engineer at the Massachusetts Institute of Technology (MIT). .
First, the innovative device cuts brain tissue into sections. It uses carefully tuned vibrations to prevent abrasion, separating cells cleanly like incredibly thin slices without displacing their junctions.
Then a chemical technique reversibly transforms these tissue sections into a flexible, expandable tissue hydrogel ripe for antibody labeling and high-resolution imaging of proteins and other internals.
Finally, a computational tool “stitches” the sliced tissues back together and maps the connections between individual cells. These “projectomes” of individual brain cells can then be integrated with profiles capturing the molecules expressed in each cell.
“We need to be able to see all these different functional components—cells, their morphology and their connectivity, subcellular architectures and their individual synaptic connections—ideally within a single brain” to be able to compare whole brains and find individual differences, Chung says.
“This technology pipeline really allows us to extract all these important functions from the same brain in a fully integrated way.”
Tissue hydrogel gently inflates tissue sections so they can be clearly visualized; and the pump continuously infuses tissues with fluorescent dyes to produce consistent staining across entire organs.
In a dizzying demonstration of the platform’s imaging capabilities, the researchers provide examples of marking one entire brain hemisphere, then zooming in to take a picture of cell circuits, followed by individual cells and their connections across junctions called synapses.
frameborder=”0″ allow=”accelerometer; automatic playback; clipboard-write; encrypted media; gyroscope; picture in picture; web-share” referrerpolicy=”strict-origin-when-cross-origin” allowfullscreen>
As for how the platform reconstructs these connections across multiple tissue slices, the computer tool has an algorithm that compares blood vessels leaving one layer and entering an adjacent layer and tracking the extension of neighboring neurons, called axons.
When they put it together, the researchers imaged the entire brains of two generous donors, one with Alzheimer’s and one without.
They revealed the usual pathological features of Alzheimer’s disease, including the accumulation of amyloid plaques and tau tangles and shrinking brain cells, but their imaging also picked up some subtler differences.
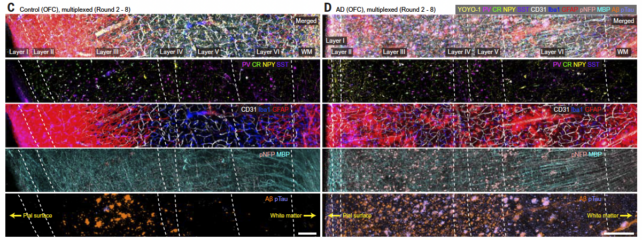
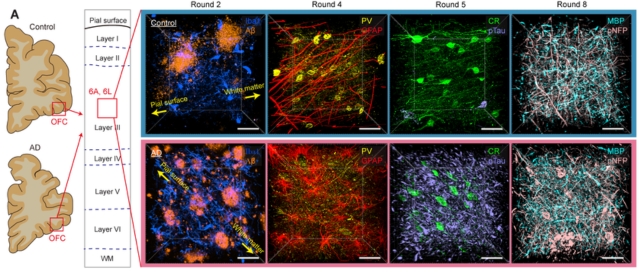
Axons of brain cells in a patient with Alzheimer’s disease were swollen. Brain cells in areas burdened with tau and amyloid proteins also lost their protective myelin sheath and withdrew from their neighbors.
This “supports neuroimaging studies that suggest severe damage to the connectivity of the orbitofrontal cortex in the late stages of Alzheimer’s disease,” the team wrote in their paper.
However, this gallery represents just one snapshot in time of just two brains.
frameborder=”0″ allow=”accelerometer; automatic playback; clipboard-write; encrypted media; gyroscope; picture in picture; web-share” referrerpolicy=”strict-origin-when-cross-origin” allowfullscreen>
Scientists have recently produced some remarkably detailed images of the human brain, zooming in on a single cubic millimeter of brain tissue – a decade-long effort that eventually produced 1.4 petabytes of data.
Imaging how the brain changes, how it slowly degenerates in diseases such as Alzheimer’s is a somewhat more difficult task, as researchers often work with postmortem brain tissue donated at the end of someone’s life or rely on traditional whole-brain scans such as MRI , and hope to detect changes before the disease manifests itself.
It’s also not yet clear how the platform might adapt to rapidly advancing advances in brain imaging, but the team is optimistic that their system will help accelerate the development of new therapies and maximize the amount of information extracted from valuable donor tissues.
“This pipeline allows us to have almost unlimited access to the tissue,” says Chung. “We can always go back and look at something new.”
The study was published in Science.