Using high-energy laser experiments, researchers have shown that magnesium oxide is likely to be the first mineral to solidify during super-Earth formation, which has major implications for the geophysical evolution of these planets.
A new study reveals that magnesium oxide, a key planet-forming mineral, may be the first to solidify as “super-Earth” exoplanets develop, with its behavior in extreme conditions significantly influencing planetary development.
For the first time, scientists have observed how atoms in magnesium oxide morph and melt under ultra-harsh conditions, providing new insight into this key mineral in the Earth’s mantle, which is known to influence planet formation.
High-energy laser experiments – which exposed tiny crystals of the mineral to heat and pressure deep inside the rocky planet’s mantle – suggest the compound could be the first mineral to solidify from magmatic oceans in the formation of “super-Earth” exoplanets. .
“Magnesium oxide could be the most important solid that controls the thermodynamics of young super-Earths,” said June Wicks, assistant professor of Earth and planetary sciences at Johns Hopkins University, who led the research. “If it has this very high melting point, it would be the first solid to crystallize as the hot rocky planet begins to cool and its interior splits into a core and a mantle.”
Implications for young planets
The findings are newly published in Scientific advances.
They suggest that the way magnesium oxide transitions from one form to another could have important implications for the factors that control whether a young planet will be a snowball or molten rock, develop water oceans or an atmosphere, or a mixture of these properties.
“In Earth-like super-Earths, where this material will be a large component of the mantle, its transformation will make a significant contribution to how quickly heat moves through the interior, which will control how the interior and the rest of the planet form and deform over time,” Wicks said. “We can think of it as representative of the interiors of these planets because it will be the material that controls their deformation, one of the most important building blocks of rocky planets.”
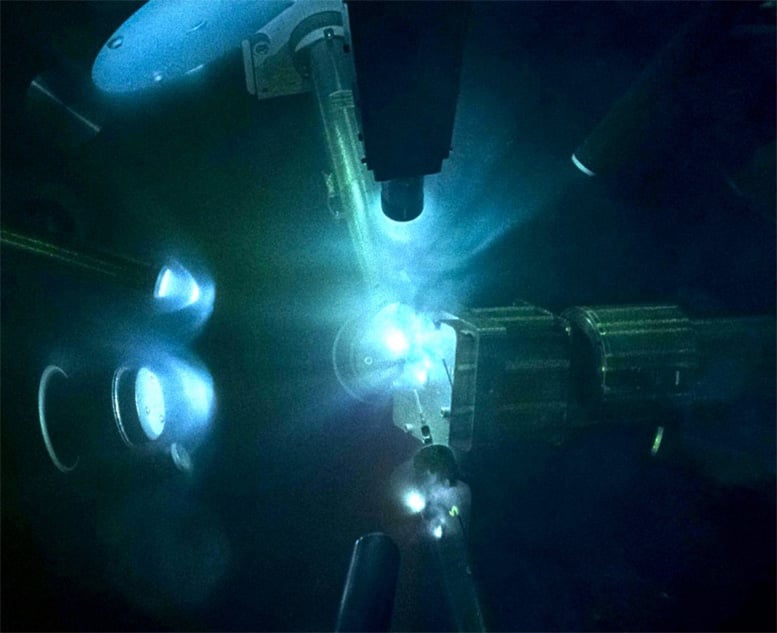
A view of laser-driven shock-compressed magnesium oxide (MgO) experiments in a chamber at the Laser Energy Laboratory. High-powered laser beams are used to compress MgO samples to pressures in excess of that at the center of the Earth. A secondary X-ray source is used to investigate the crystal structure of MgO. Brighter regions are glowing plasma emissions on nanosecond time scales. Credit: June Wicks/Johns Hopkins University
Bigger than Earth, but smaller than the giants Neptune or Uraniumsuper-Earths are key targets in exoplanet is looking for because they are commonly found among other solar systems in the galaxy. While the composition of these planets can vary from gas to ice or water, rocky super-Earths are expected to contain significant amounts of magnesium oxide, which can affect the planet’s magnetic field, volcanism and other key geophysics as it does on Earth, Wicks said. .
To mimic the extreme conditions this mineral might have endured during planet formation, Wick’s team subjected small samples to ultra-high pressures using the Omega-EP laser facility at the University of Rochester’s Laser Energetics Laboratory. The researchers also shot X-rays and recorded how those light beams bounced off the crystals to watch how their atoms rearranged in response to increasing pressures, specifically noting at what point they transformed from a solid to a liquid.
When compressed extremely hard, the atoms of materials like magnesium oxide change their arrangement to sustain the crushing pressures. This is why the mineral changes from a rock salt “phase” resembling table salt to another configuration, such as that of another salt called cesium chloride, as the pressure increases. This leads to a transformation that can affect the mineral’s viscosity and impact on the planet as it matures, Wicks said.
Stability of magnesium oxide at high pressures
The team’s results show that magnesium oxide can exist in both of its phases at pressures ranging from 430 to 500 gigapascals and temperatures around 9,700 Kelvin, nearly twice that of the surface of the Sun. Experiments also show that the highest pressures the mineral can withstand before completely melting are greater than 600 gigapascals, about 600 times the pressure a person would feel in the deepest ocean trenches.
“Magnesium oxide melts at a much higher temperature than any other material or mineral. Diamonds may be the hardest materials, but they are the last to melt,” Wicks said. “When it comes to extreme materials on young planets, magnesium oxide is likely to be solid, while everything else hanging down there in the mantle will turn to liquid.”
The study shows the stability and simplicity of magnesium oxide under extreme pressures and could help scientists develop more accurate theoretical models to investigate key questions about the behavior of this and other minerals on rocky worlds like Earth, Wicks said.
“This study is a love letter to magnesium oxide, because it’s amazing that it has the highest melting point we know of — at pressures beyond the center of the Earth — and still behaves like regular salt,” Wicks said. “It’s just a beautiful, simple salt, even at these record pressures and temperatures.”
Reference: “B1-B2 transition in shock-compressed MgO” by June K. Wicks, Saransh Singh, Marius Millot, Dayne E. Fratanduono, Federica Coppari, Martin G. Gorman, Zixuan Ye, J. Ryan Rygg, Anirudh Hari, Jon H. Eggert, Thomas S. Duffy, and Raymond F. Smith, 07 Jun 2024, Scientific advances.
DOI: 10.1126/sciadv.adk0306
Additional authors are Saransh Singh, Marius Millot, Dayne E. Fratanduono, Federica Coppari, Martin G. Gorman, Jon H. Eggert, and Raymond F. Smith of Lawrence Livermore National Laboratory; Zixuan Ye and Anirudh Hari of Johns Hopkins University; J. Ryan Rygg of the University of Rochester; and Thomas S. Duffy of Princeton University.
This research was supported by the NNSA through the National Laser Users’ Facility Program under Contract Nos. DE-NA0002154 and DE-NA0002720 and the Laboratory Directed Research and Development Program at LLNL (Project No. 15-ERD-012). This work was performed under the auspices of the US Department of Energy Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344. The research was supported by the National Nuclear Security Administration through the National Laser Users’ Facility Program (Contract Nos. DE-NA0002154 and DE-NA0002720) and the Laboratory-Directed Research and Development Program at LLNL (Project Nos. 15-ERD-014, 17 -ERD- 014 and 20-ERD-044).