Scientists have captured the first case of an embryo forming at an early stage, which could help solve the “mystery” of how congenital birth defects start in humans.
Australian scientists watched in amazement as the quail embryo’s cells crawled around its protein-based support structure – organizing into the earliest form of a heart and the first stage of its spine and brain, called the ‘neural tube’.
An innovative technique using a fluorescent protein was used to illuminate these cells in the tiny embryo as the team watched its early moments take shape.
Because of the quail embryo’s similarity to the human species at these early stages, scientists now plan to study in real time what early missteps these embryonic cells lead to birth defects to help improve future human treatments.
For the first time ever, scientists have captured real-time video of an early-stage embryo forming the “neural tube” that will grow to become its brain and spinal cord (see above). An innovative technique using a fluorescent protein was used to illuminate this tiny embryo
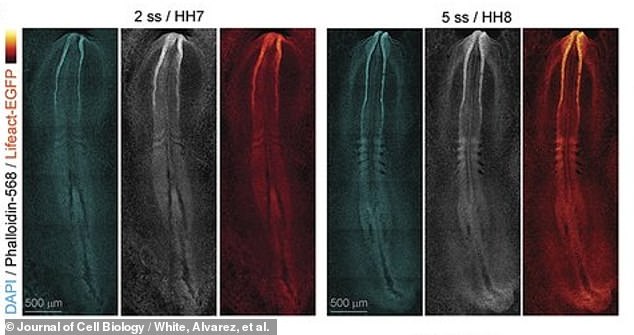
The researchers – molecular bioscientists from the University of Queensland in Australia – report that these new videos could soon help modern medicine understand congenital birth defects and how to correct them. Above, images of the early spine and brain formation of the embryo
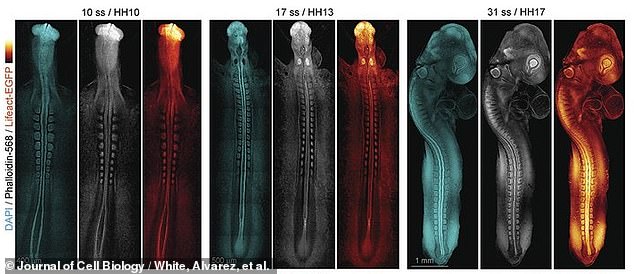
Above, later still images of early spinal cord and embryonic brain formation
About three percent of all human babies are born with congenital birth defects, the study’s lead author said, most commonly heart defects and neural tube defects.
The only treatment available is surgery, which is performed just days after birth, but in more severe cases, heart defect transplants may be needed.
Researchers at the University of Queensland created a genetically engineered quail embryo that formed while producing a reflective fluorescent protein called Lifeact.
The genes for making these Lifeact proteins were implanted into a living quail embryo by direct injection into its primordial germ cells circulating in the blood.
“Leaflet.” [meaning birds, like quail] embryos are an excellent model of human development,” says Dr. Melanie White, but especially in these early stages of growth.
“The development of many major organs including the heart and the neural tube (which goes on to form the brain and spinal cord) is very similar,” she said.
Quail embryos are also easier to record alive when they are growing because the thin shell of the egg is easier for medical technology to see and leave undisturbed.
“It is very difficult to film these stages of embryonic development as they occur after human embryos are implanted in the mother’s womb,” explained Dr. White.
“Because quail grow in the egg, they are very accessible for imaging,” she noted, “and their early development is very similar to that of humans by the time [human] the embryo implants in the uterus.’
The glow of fluorescent proteins above revealed the embryo’s early scaffolding, called the “actin cytoskeleton,” which gives cells shape and helps them move. Fluorescent proteins bind selectively to actin, also a protein that gives definition to this early embryonic structure
The glow of these fluorescent proteins revealed the embryo’s early protein scaffolding called the “actin cytoskeleton”—which gives cells a shape to attach to and helps them move.
These fluorescent proteins bind selectively to actin, which is also a protein, lighting up and giving definition to this early embryonic structure.
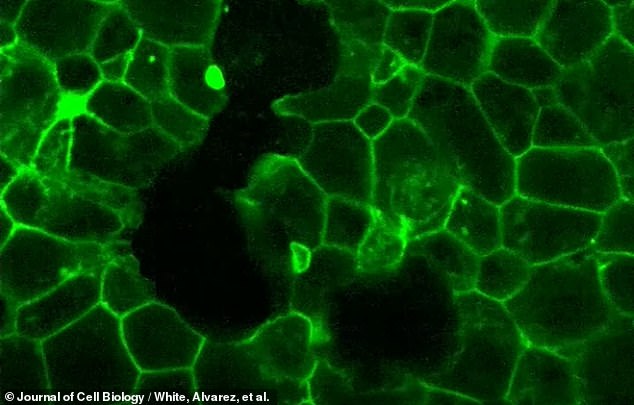
With this illumination, the scientists were able to record the formation of arm-like projections on individual cells (lamellipodia and filopodia), which help the cells crawl along the protein supports of the cytoskeleton to the correct location.
Dr. White and her colleagues documented cardiac stem cells deep within the embryo as they climbed into position on this cytoskeleton to form the early heart.
“This is the first time anyone has captured the cellular actin cytoskeleton facilitating this contact in live imaging,” Dr. White said in a statement.
“One of the key things we’re missing is dynamic information about how the embryo coordinates the movement, positioning and fate of its cells as it moves from one stage to the next,” Dr White explained the purpose of the new videos to Newsweek.
“This information can only be obtained using live imaging approaches where we can watch how the embryonic tissue changes over time,” she said.
“How cells interact and move in real time to organize themselves into complex tissues in the forming embryo is still largely a mystery,” says Dr. White.
One of the other crucial events documented by the Queensland team’s technique was the “zipping” of cells along the long open edges of the embryo’s neural tube.
Like a burrito or wrap, the cells fold into this tube-like shape and close into the tube in a zipper-like motion as the cells’ lamellipodia and filopodia come together.
Once closed, this newly formed neural tube will continue to grow and mature into its future form as the brain and spinal cord.
“We saw the cells reach through the open neural tube with their projections to make contact with the opposite side,” Dr. White said.
“The more protrusions the cells made, the faster the tube turned on.”
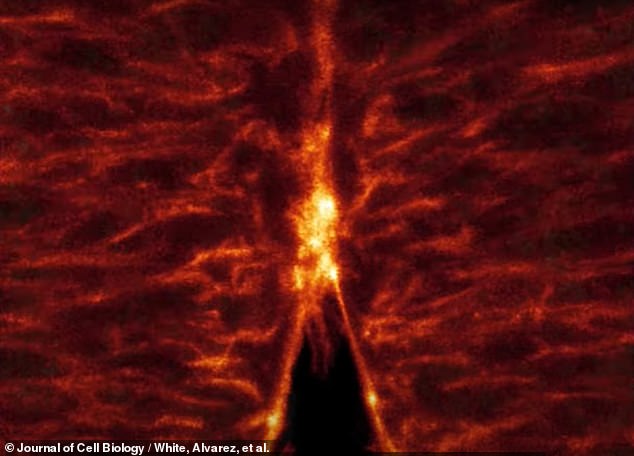
The image above shows the ‘zipper’ movement as the ‘neural tube’ of the embryo is formed by the shoulder processes of each cell – its lamellipodia and filopodia – grasping each other.
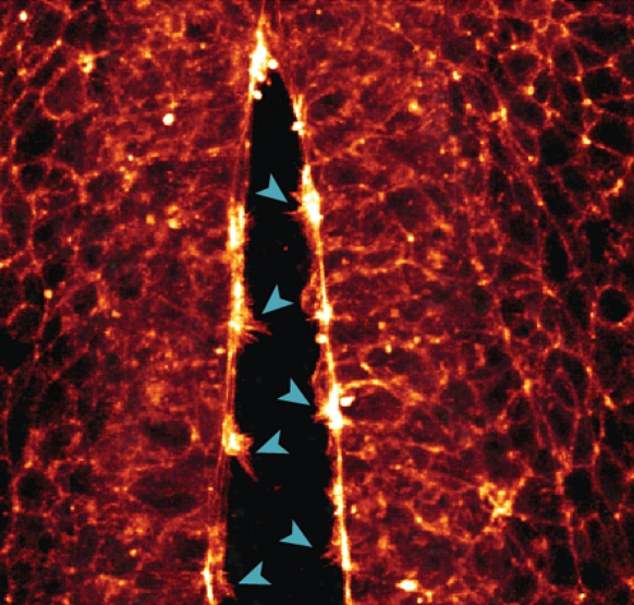
Above, the scientists were able to record the formation of arm-like protrusions on individual cells – which help the cells crawl along the cytoskeleton’s protein supports to the correct location. In the image above, the arms of the cell come together to close the walls of the neural tube.
It is precisely this process, she said, that often “goes wrong or is disrupted” during the fourth week of human development — leading to congenital brain or spinal cord defects, whether inherited or caused by environmental factors.
“Our goal is to find proteins or genes that can be targeted or used to screen for congenital birth defects in the future,” Dr White said.
“We are very excited about the possibilities that this new quail model now offers to study development in real time,” said the researcher, who also leads the Dynamics of Morphogenesis Laboratory at the Queensland Institute of Molecular Biosciences.
The work of Dr. White and her team was published this June in the Journal of Cell Biology.
“In our lab, we are now building on the initial experiments we did to try to understand how the heart and neural tube form in real time,” she said.
Specifically, the team of Dr. Whitea is now “studying how mutations found in patients or maternal factors (diabetes, nutritional deficiencies) disrupt this development and lead to birth defects.