Researchers pioneered a method of growing diamonds under low pressure and temperature, which revolutionized the traditional process of producing synthetic diamonds and expanded the possibilities of scientific and technological progress.
Scientists have created a new system of liquid metal alloys to produce diamonds under mild conditions.
Did you know that 99% of synthetic diamonds are produced using high pressure and high temperature (HPHT) methods? The general view is that diamonds can only be grown using liquid metal catalysts at pressures of 5-6 gigapascals (about 50,000-60,000 atmospheres) and temperatures between 1300-1600°C. However, the size of diamonds produced by HPHT is typically limited to about one cubic centimeter due to process limitations.
This means that such high pressures can only be achieved on a relatively small length scale. The discovery of alternative methods of producing diamonds in liquid metal under milder conditions (especially at lower pressures) is an interesting basic science challenge that, if achieved, could revolutionize diamond production. Could the prevailing paradigm be challenged?
A team of researchers led by Director Rod Ruoff at the Center for Multidimensional Carbon Materials (CMCM) within the Institute for Basic Science (IBS), including graduate students at the Ulsan National Institute of Science and Technology (UNIST), grew diamonds under the conditions. pressure of 1 atmosphere and at 1025 °C using liquid metal alloy consists of gallium, iron, nickel and silicon, thereby breaking the existing paradigm. The discovery of this new growth method opens up many possibilities for further basic science studies and for accelerating the growth of diamonds in new ways.
Increasing the efficiency of the experiment
Director Ruoff, who is also a UNIST Distinguished Professor, notes, “This groundbreaking breakthrough was the result of human ingenuity, unrelenting effort, and the collaborative efforts of many collaborators.” Researchers led by Ruoff conducted a series of experiments involving several hundred parameter adjustments and a variety of experimental approaches before finally succeeded in growing diamonds using a “homemade” cold wall vacuum system.
Ruoff notes: “We performed our parametric studies in a large chamber (named RSR-A with an internal volume of 100 liters) and our search for parameters that would provide diamond growth was slowed down by the time required to pump out the air (about 3 minutes), flush with inert with gas (90 minutes), then pumped down again to the vacuum level (3 minutes) in order to fill the chamber with a pressure of 1 atmosphere of a completely pure mixture of hydrogen and methane (again 90 minutes); that’s more than 3 hours before the experiment could start! I asked Dr. Won Kyung SEONG to design and build a much smaller chamber that would greatly reduce the time required to start (and finish!) a liquid metal experiment exposed to a mixture of methane and hydrogen.” Seong adds, “Our new home-built system (named RSR-S , with an internal volume of only 9 liters) can be pumped out, cleaned, pumped out and filled with a mixture of methane and hydrogen in a total time of 15 minutes. Parametric studies were greatly accelerated and this helped us discover the parameters for which diamond grows in liquid metal!’
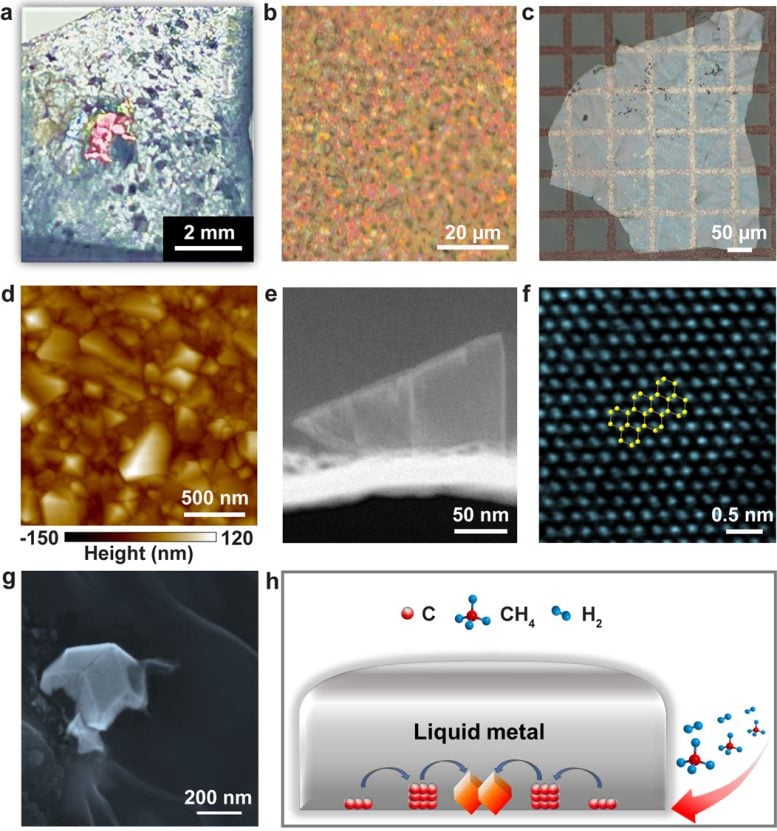
Diamond growth in a liquid metal alloy under 1 atmosphere pressure. (a) Photograph showing a grown diamond on the surface of a solidified liquid metal. (b) Optical image of a grown continuous diamond film on a solidified liquid metal surface. (c) Optical image of a transferred diamond film on a Cu TEM grid coated with a Quantifoil amorphous carbon film. (d) Atomic force microscopy topography image of a transferred diamond film on a Cu TEM grid. (e) TEM image of a cross-section of a single diamond particle grown on a solidified liquid metal surface. (f) Atomic resolution TEM image of as-grown diamond. (g) Scanning electron microscopy image showing a grown diamond (partially) immersed in solidified liquid metal. (h) Schematic showing carbon diffusion leading to diamond growth on the lower surface of the liquid metal. Credit: Institute for Basic Science
The team discovered that diamond grows on the subsurface of a liquid metal alloy consisting of a 77.75/11.00/11.00/0.25 (atomic percent) gallium/nickel/iron/silicon mixture when exposed to methane and hydrogen under pressure 1 atm at ∼1025 °C.
Yan GONG, a UNIST graduate student and first author, explains: “One day with the RSR-S system, when I did an experiment and then cooled the graphite crucible to solidify the liquid metal and removed the solidified piece of liquid metal, I noticed: a rainbow pattern ” spread over several millimeters on the underside of this piece. We discovered that the rainbow colors are due to diamonds! This allowed us to identify the parameters that supported reproducible diamond growth.
Initial formation occurs without the need for diamond or other seed particles commonly used in conventional HPHT and chemical vapor deposition synthesis methods. Once formed, the diamond particles bond together to form a film that can be easily detached and transferred to other substrates for further studies and potential applications.
Synchrotron two-dimensional X-ray diffraction measurements confirmed that the synthesized diamond film has a very high purity of the diamond phase. Another interesting aspect is presence silicon free color centers in the diamond structure, an intense zero-phonon line was found at 738.5 nm in the photoluminescence spectrum excited using a 532 nm laser.
Co-author Dr. Meihui WANG notes, “This synthesized diamond with colored silicon centers can find applications in magnetic sensing and quantum computing.”
Mechanisms and theoretical perspective
The research team took a deep dive into the possible mechanisms of diamond nucleation and growth under these new conditions. High-resolution transmission electron microscopy (TEM) imaging of cross-sections of the samples showed a roughly 30-40 nm thick amorphous subsurface region in the solidified liquid metal that was in direct contact with the diamonds. Co-author Dr. Myeonggi CHOE notes, “About 27 percent of the atoms that were present on the top surface of this amorphous region were carbon atoms, with the carbon concentration decreasing with depth.”
Ruoff explains: “The presence of such a high concentration of ‘dissolved’ carbon in a gallium-rich alloy might be unexpected, as carbon is reported to be insoluble in gallium. This may explain why this region is amorphous – while all other regions of the solidified liquid metal are crystalline. This subsurface area is where our diamonds nucleate and grow, which is why we focused on it.
The researchers exposed the Ga-Fe-Ni-Si liquid metal to methane/hydrogen for a short period of time to try to understand the early stage of growth – before a continuous diamond film is formed. They then analyzed carbon concentrations in subsurface regions using time-of-flight secondary ion mass spectrometry depth profiling. After 10 minutes of running, no diamond particles were visible, but ~65 at% carbon atoms were present in the region where diamond usually grows. Diamond particles began to be found after 15 minutes of running and there was a lower subsurface C atom concentration ~27 at%.
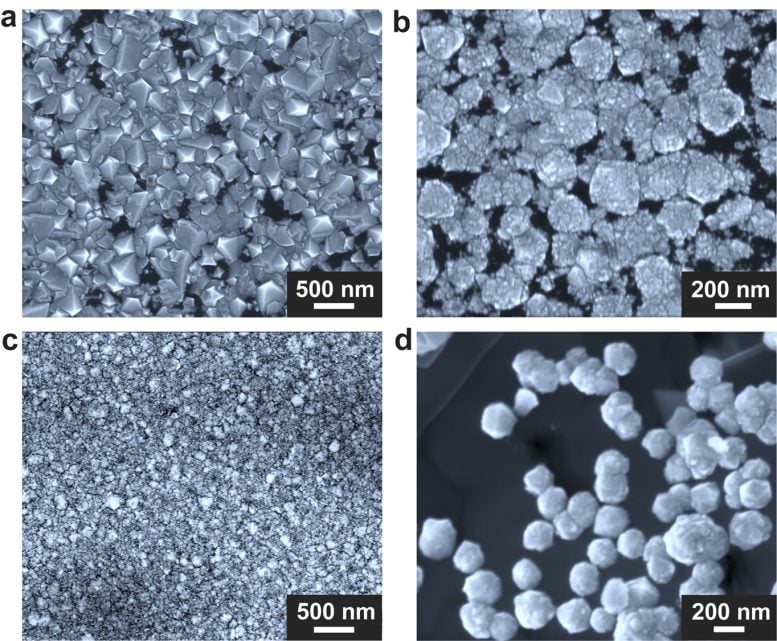
Diamonds of different morphologies grown under different growth conditions. (a) Growth using a Ga/Ni/Fe/Si liquid metal alloy (77.75/11.00/11.00/0.25 at%) under methane/hydrogen (molar ratio 1/20). (b) Growth using liquid metal alloy Ga/Ni/Fe/Si (77.50/11.00/11.00/0.50 at%) under methane/hydrogen (molar ratio 1/20). (c) Growth using liquid metal alloy Ga/In/Ni/Fe/Si (38.88/38.87/7.33/14.67/0.25 at%) under methane/hydrogen (molar ratio 1/ 20). (d) Growth using a Ga/Ni/Fe/Si liquid metal alloy (77.75/11.00/11.00/0.25 at%) under methane/hydrogen (molar ratio 1/5). Credit: Institute for Basic Science
Ruoff explains, “The concentration of subsurface carbon atoms is so high around 10 minutes that this time exposure is close to or in supersaturation, leading to nucleation of diamonds either in 10 minutes or sometimes between 10 and 15 minutes. The growth of diamond particles after nucleation is expected to occur very quickly, sometimes between 10 and 15 minutes.
The temperature at 27 different locations in the liquid metal was measured using a growth chamber attachment with an array of nine thermocouples designed and manufactured by Seong. The central region of the liquid metal was found to be at a lower temperature compared to the corners and sides of the chamber. This temperature gradient is thought to be what drives the diffusion of carbon towards the central region, facilitating diamond growth.
The team also found that silicon plays a key role in this new diamond growth. As the concentration of silicon in the alloy increases from the optimum value, the size of the grown diamonds decreases and their density increases. Diamonds could not be grown at all without the addition of silicon, suggesting that silicon may be involved in the initial nucleation of diamond.
This was supported by various theoretical calculations performed to reveal the factors that may be responsible for the growth of diamonds in this new liquid metal environment. The researchers found that silicon promotes the formation and stabilization of certain carbon clusters by predominantly forming sp3 bonds like carbon. It is thought that small carbon clusters containing Si atoms could serve as “pre-nuclei”, which can then grow further to form diamond seeds. The likely size range of the initial nucleus is thought to be around 20 to 50 C atoms.
Ruoff says: “Our discovery of diamond nucleation and growth in this liquid metal is fascinating and offers many exciting opportunities for more basic science. We are now investigating when nucleation occurs and thus the rapid subsequent growth of the diamond. Also, ‘temperature drop’ experiments, where we first supersaturate carbon and other necessary elements, followed by a rapid reduction in temperature to trigger nucleation, are some studies that look promising to us.”
The team found that their growth method offered significant flexibility in the composition of the liquid metals. Researcher Dr. Da LUO notes, “Our optimized growth was achieved using a liquid gallium/nickel/iron/silicon alloy. However, we also found that high-quality diamond can be grown by replacing nickel with cobalt, or by replacing gallium with a mixture of gallium and indium.”
Ruoff concludes: “Diamond can be grown in a wide range of alloys with relatively low melting points of liquid metals, for example containing one or more of indium, tin, lead, bismuth, gallium and potentially antimony and tellurium – and including other alloys in the molten alloy. elements such as manganese, iron, nickel, cobalt and so on as catalysts and others as additives that provide color centers. And in addition to methane (various gases as well as solid carbons), a wide variety of carbon precursors are available. New designs and methods for introducing carbon atoms and/or small clusters of carbon into liquid metals for diamond growth will certainly be important, and the creativity and technical ingenuity of the global research community, based on our discovery, seems likely to me to quickly lead to other related approaches and experimental configurations . There are many interesting paths to explore!”
Reference: “Growth of diamond in liquid metal at 1 atm pressure” by Yan Gong, Da Luo, Myeonggi Choe, Yongchul Kim, Babu Ram, Mohammad Zafari, Won Kyung Seong, Pavel Bakharev, Meihui Wang, In Kee Park, Seulyi Lee, Tae By Joo Shin, Zonghoon Lee, Geunsik Lee, and Rodney S. Ruoff, 24 Apr 2024, Nature.
DOI: 10.1038/s41586-024-07339-7